Pipeline
Treventis has ongoing oligomer inhibitor programs in several CNS disease areas. The company is expanding its CCM platform into other indications including in oncology and other neurodegenerative disorders.
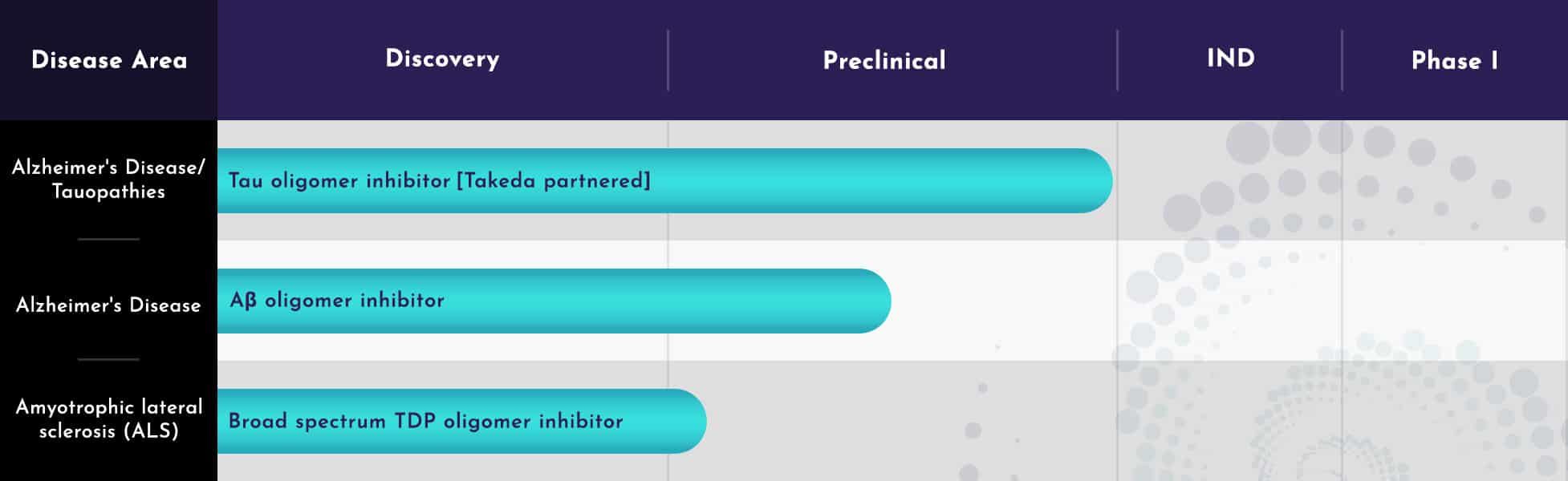
Tau and Aβ oligomer inhibitor programs
TREVENTIS’ lead program concerns design of small molecule anti-oligomerization agents in Alzheimer’s and tauopathies using our in silico Common Conformational Morphology (CCM) models of the protein misfolding process. Our process has involved the construction of in silico models of misfolded tau, followed by in vitro testing of the best-predicted compounds; and our experimental hit rate is much higher than would be expected by chance. Surface plasmon resonance, TRESI-HDX and 2D NMR also provides structural support for the models.
The hit compounds identified in our innovative primary screen of oligomerization have been optimized to demonstrate potent anti-oligomer effects on misfolded proteins, either broadly or selectively from structure-activity relationships. We have made over 1,800 small molecules among several classes designed to have drug-like characteristics. Advanced compounds in this series have demonstrated oral bioavailability and are not promiscuous inhibitors of protein-protein interactions (as shown through dynamic light scattering, tubulin assembly, etc.). Moreover, compounds in the series have shown target engagement in vivo against tau, with pharmacologically relevant in vivo potency.
In 2023, Treventis entered into an option, collaboration, and license agreement with Takeda for the further research, development, and commercialization of potential candidate small molecules in this program.
Treventis has a separate, unpartnered small molecule program internally for the inhibition of Aβ oligomerization, leveraging the additional drug classes and small molecules in the Treventis library to accelerate drug development. This innovative program represents a differentiated mechanism of action from that of recently approved anti-amyloid antibodies, with the program goal to supply a drug with similar efficacy as an antibody but avoid Amyloid Related Imaging Abnormalities that are commonly seen with such antibody approaches.
Broad spectrum TDP oligomer inhibitor
This program aims to develop a therapeutic for halting disease progression in Amyotrophic Lateral Sclerosis (ALS), by designing small molecules that inhibit the misfolding of TAR DNA-binding protein (TDP) isoforms in ALS and thereby halt further growth and pathology of neurotoxic aggregates.
Compounds experimentally assayed to date from the TREVENTIS compound collection have been identified as having anti-TDP oligomer activity in both turbidity and cell-based models. Three chemotypes have been identified that show favorable activity profiles. Many compounds show a profile of binding to all three TDP isoforms, which holds promise as a broad-spectrum anti-misfolding agent for TDPopathies.
Beyond ALS, this program is also of relevance to frontotemporal dementia (FTD) mediated by TDP. In 2022, Treventis received a $2.97M grant from the U.S. Department of Defense to support Treventis’ development of drugs against FTD.
p53 oligomer inhibitor
p53 is the most frequently mutated target in human cancer, with mutations found in >50% of all tumors that have a deleterious effect on it and other related tumor suppressors. This cancer susceptibility gene is the subject of significant pharmacological interest, with most effort focused on developing reactivators of mutant p53 in cancers. Comparatively less studied is the aggregation and misfolding of mutp53 protein. Mutations in p53 result in the aggregation of the protein, which can itself acquire oncogenic GOF along with sequestering functional wt p53 and other tumor suppressors (e.g., p63 and p73).
Using TREVENTIS technologies we have identified several classes of drug-like compounds that inhibit mutP53 oligomerization in a dose-dependent manner. Our preliminary results in cells demonstrated that half of those hits inhibited cell growth of mutant p53 cells, but little toxicity on wt cells. We are moving these compounds forward to further discovery efforts in this exciting new area. In 2023, Treventis received a grant of $489,738 from the U.S. Department of Defense to further explore anti-mutP53 targeting small molecules.